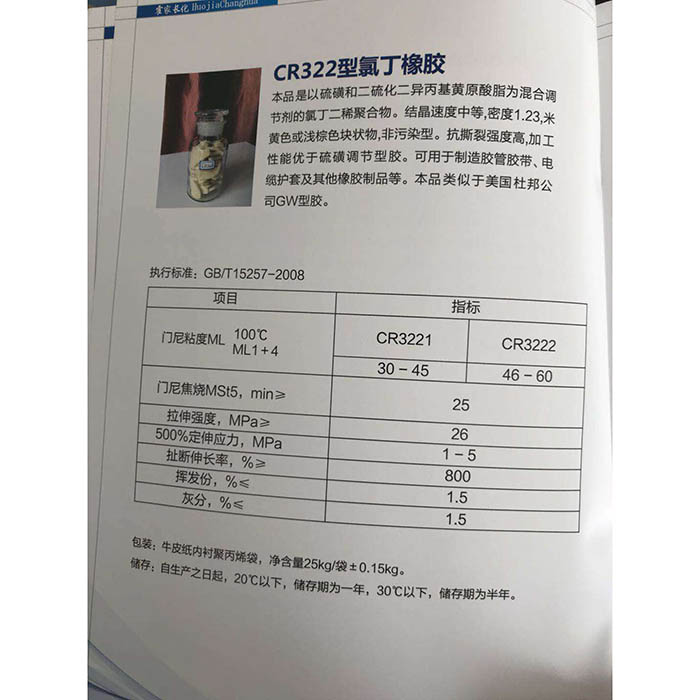
konutai waihā
konutai waihā, ko tana tauira matū ko NaOH, e mohiotia ana ko te houra, te houra me te houra. Ka rewa, ka puta te haukinia. He puia kahaalkali, i te nuinga o te waa he tawerewere, he kirikiri ranei. He ngawari te wairewa ki te wai (ka rewa ki te wai, ka puta te wera) ka hanga he wairewa kawakore. I tua atu, he deliquescence me te ngawari ki te ngongo i te kohu wai (deliquescence) me te hauhā (deterioration) ki te hau. Ko te NaOH tetahi o nga matū e tika ana i roto i nga taiwhanga matū, a ko tetahi o nga matū noa. Ko te hua parakore he karaehe, he karaihe maramara. Kiato 2.130 g/cm. Ira whakarewa 318.4℃. Ko te 1390 ℃ te ira kohua. Kei roto i nga hua ahumahi he iti te konutai konutai me te konutai warowaiha, he tioata ma me te puatakore. He paraka, he kirikiri, he kirikiri me te ahua rakau. Momo rahinga 40.01
konutai waihāka taea te whakamahi hei kaihoko horoi kawakore i roto i te maimoatanga wai, ka rewa i roto i te waihano me te glycerol; Karekau ki te propanol me te ether. Ka pirau hoki te waro me te konutai i te wera nui. Te tauhohenga whakawehenga me te halogen penei i te chlorine, bromine me te iodine. Whakakorehia ki te waikawa kia hanga te tote me te wai.
Nga ahuatanga o te kopa
Ko te konutai waikawa he totoka kiriatairangi ma. Ko tana wairewa wai he reka astringent me te kare o te satiny.
Kopa kopa He deliquescence i te rangi.
Whakakopa wai
Ko te kawakore totoka he tino waikore. Ka pa ana ki te hau, ka mimiti i nga ngota ngota wai i te rangi, ka mutu ka memeha katoa ki te otinga, engari karekau he hygroscopicity te konutai konutai wai.
Wairewa takai
Kawakoretanga takai
Ko te konutai waiwai ka wehe katoa ki nga katote konutai me nga katote hauwai ka rewa ki te wai, no reira ko te tikanga o te alkali.
Ka taea e ia te kawe i te tauhohenga whakaheke waikawa-papa ki tetahi waikawa protonic (no te tauhohenga pirau rua ano hoki):
NaOH + HCl = NaCl + H₂O
2NaOH + H₂SO₄=Na₂SO₄+2H₂O
NaOH + HNO₃=NaNO₃+H₂O
Waihoki, ka taea e tana otinga te tauhohenga pirau rua ki te wairewa tote:
NaOH + NH₄Cl = NaCl +NH₃·H₂O
2NaOH + CuSO₄= Cu(OH)₂↓+ Na₂SO₄
2NaOH+MgCl₂= 2NaCl+Mg(OH)₂↓
Tauhohenga saponification takai
I roto i te maha o nga tauhohenga waro, he rite ano te mahi a te konutai wai hei whakakorikori, i roto i enei ko te tino tohu ko te saponification:
RCOOR' + NaOH = RCOONa + R'OH
Tiango etahi
Ko te take ka ngawari te kino o te konutai hauwai ki te konutai warowaikawa (Na₂CO₃) i te rangi na te mea kei roto i te hau te hauhā (co):
2NaOH + CO₂ = Na₂CO₃ + H₂O
Mēnā ka whakaurua te hauhā hauhā nui, ka puta te konutai pākawakawa (NaHCO₃), e mōhiotia whānuitia ana ko te houra tunutunu, ā, ko te whārite tauhohenga e whai ake nei:
Na₂CO₃ + CO₂ + H₂O = 2NaHCO₃
Waihoki, ka taea e te konutai hauwai te tauhohe ki nga waikura waikawa penei i te hauhaa hika (SiO₂) me te whanariki hauhā (SO):
2NaOH + SiO₂ = Na₂SiO₃ + H₂O
2 NaOH+SO (trace) = Na₂SO₃+H₂O
NaOH+SO₂ (tawhetawhe) = NaHSO₃ (NASO hangaia me te wai ka tauhohe ki te SO nui ki te whakaputa nahSO)